“Doctor, do these medicines have side effects?”
This is a common question that patients ask their doctor. This article explains the concept of side effects in some detail, and provides practical strategies to reduce such risks.
What are side effects?
When we use a medicine to treat an illness, we are interested in its effect specifically on the affected area of the body. However, any medicine will have more than one effect on the body, some of which are unwanted. These are termed side effects or adverse drug reactions.
For example, when we take pills to control a runny nose, we may also become sleepy in the process, as antihistamines commonly cause drowsiness as a side effect. Likewise, while going to the beach to wet our feet in the sea, some sand sticks to our feet when we return, whether we like it or not. Though the water was our target, the sand became the side effect.
Read: Everyday Health | Hepatitis B: an ounce of prevention is worth a pound of cure
The WHO defines adverse drug reaction (ADR) as a noxious and unintended response occurring during treatment using normal doses. The term adverse event (AE) is used when the reported event is not yet causally linked with the drug.
Fortunately, most side effects are mild, and disappear after stopping the drug.
Why are so many side effects listed for every medication?
Most medications will have several side effects listed in the product information, but this does not mean that everyone who takes these medications will suffer those side effects, or that the medication is harmful.
To understand this, it is useful to know the basic steps of new drug development.
When a pharmaceutical company develops a new medication, they first submit a new drug application (NDA) to the FDA (Food and Drug Administration). Laboratory and animal studies are first done. After confirming safety in animals, the manufacturer will then file an investigational new drug (IND) application with the FDA prior to testing in human subjects. Phase 1, 2 and 3 clinical trials are then performed in humans, to confirm that the medication is clinically effective and safe. It is a requirement during this process to monitor and list the observed side effects correctly and in sufficient detail, failing which the FDA will not approve it for use.
To conceptualize this better, let us consider the example of a bicycle. When we purchase a bicycle from a shop, we should ideally be made aware that:
1. Reckless use of the bicycle can result in a fall, leading to severe head injury and limb fractures
2. Pancreatic injury can happen from handlebar trauma
3. The brake cable can fail without warning, resulting in potentially fatal accidents
4. The brake will not work in wet conditions
5. The chain might slip, resulting in temporary immobilization
6. The tire can suffer a puncture, causing delays during a journey
7. Using the cycle at night without lights can invite accidents
8. Protective equipment such as bicycle helmet (mandatory in developed countries) and knee caps must be worn to reduce the risk of injury during a fall
9. Wrong seating angle can lead to back pain, and so on.
However, in India, we do not typically get briefed like this when we buy a bicycle. The case of starting a new medication is no different. Just as a simple bicycle has its own share of side effects, all medications have side effects, though most of us would rather not hear about them.
Why do some people say there are no side effects to certain treatments?
While it is conventional in the system of modern medicine to list every observed side effect in the product monograph, it is not so for alternative systems of medicine, which typically will list the ingredients and directions for use on the product label, but not the side effects.
What about side effects that are discovered after approval?
This is a problem with drugs newly approved by the FDA. Despite earnest efforts by the drug development team, not all side effects would have become known at the time of approval. Even though each drug gets tested on an average of 1,500 people, it still might not be enough for all the side effects to manifest, as some serious events occur only once in 20,000 or 50,000 people. Hence, it is important for practising doctors to be vigilant for any unusual effects after prescribing a newly approved drug, and report such events promptly to monitoring agencies. In India, these events are to be reported to the nearest medical college having a pharmacovigilance center.
Read: Everyday Health | Fatty liver: are you at risk of cirrhosis?
Once such an event gets reported, experts will study the case and determine whether the side effect reported was genuinely caused by the new drug, or an incidental and unrelated event. They do so by reviewing all the reports that came in about this drug, and analyzing among other parameters, the signal-to-noise ratio. ‘Noise’ refers to most of the reports that turn out to be false alarms, while ‘signal’ indicates a true adverse drug reaction.
It is such vigilance by practising doctors that helped identify several serious side effects that resulted in the withdrawal of potentially unsafe medications from the market. For instance, terfenadine, a popular antihistamine, was withdrawn from the market after reports of a dangerous heart condition called torsades de pointes came in.
Let us now look at the mechanisms of side effects in some detail.
Most of the observed side effects (adverse drug reactions) occur because of the direct action of the new medication on the body. They may also occur from disagreement between the new drug and another medication that the person is already on – a phenomenon also called ‘drug interaction’. It is impractical to describe all the mechanisms of adverse drug reactions, but the following examples will help gain some insight into this complex topic.

Allergic reactions: Allergy is a common type of side effect, due to the body’s immune system launching an unusually aggressive response against the new drug. Allergic reactions can be mild and harmless in the form of an itchy rash, or moderate in severity such as wheezing.
Rarely, they can be serious and life-threatening in the form of anaphylactic shock, a dramatic condition that results in sudden severe breathing difficulty and collapse. These can’t always be predicted. Penicillin injections cause anaphylactic reactions in a tiny number of people – approximately 1 in 10,000. This is due to pre-existing IgE-type anti-penicillin antibodies and should not be confused with the relatively common penicillin allergy, which is not serious.
Pain killers, anaesthetic agents and latex are associated with anaphylaxis. Anaphylactic reactions can also occur to peanuts, prawns or bee-stings. It could turn fatal in a matter of minutes unless treatment is administered immediately. Injection of adrenalin into the thigh muscle is the first step, followed by other measures. Doctors try to reduce the risk of anaphylaxis by administering a small test dose into the skin first, and then observing for any reaction. Rarely, anaphylaxis can occur even with a test dose.
Non-allergic side effects: Most medications are substances that are not naturally produced in the body, but cause actions in the body that help with alleviating disease. It is almost impossible to produce a medicine that works only in one part of the body, as most body cells are similar in basic structure and functioning, although each cell will have additional specialized roles. For example, a liver cell and a brain cell share the same structural template, but they have evolved to perform totally different tasks in the body. Cells and organ systems are connected with a vast network of blood vessels, nerves, and complex molecular signalling feedback systems. It follows that a medication that works in one area will have some effect in other organ systems too.
For instance, aspirin was invented as a pain-killer due to its ability to block the production of pain-causing molecules called prostaglandins. It was then observed that those people who took aspirin also bled more easily. Researchers later found that prostaglandins by themselves are a large family of molecules with multiple actions involving more than one organ system -- they were not just involved with pain. It was soon discovered that by blocking the action of prostaglandins, aspirin impaired the functioning of platelets that are involved with blood clotting. It reduced the tendency of blood to clot inside blood vessels, which is a property that was later used for its protective effect on the heart. Prostaglandins are also involved with normal kidney and gut function; blocking their actions can sometimes lead to kidney failure and stomach bleeds.
Thus, one can say that the side effect of aspirin became useful for another disease condition like heart disease and stroke, but also caused unexpected illness in a few people.
Digoxin, a plant extract, was found to improve the pumping action of the heart, but its actions on the gut can lead to vomiting, while its actions on the brain produce dizziness. It can also cause dangerous rhythm problems of the heart, especially when the blood level of potassium is low. Low potassium levels can occur as a result of vomiting, and also with the use of other medications such as frusemide that are used in heart failure. This is an example of one drug setting up the scene for toxicity of another.
The above examples illustrate the three-dimensional complexity of the subject of adverse drug reactions. The principles outlined in these cases hold true for most medications that are used on the body, regardless of whether they are chemical or herbal in origin.
The role of liver and kidney in adverse drug reactions (ADR)
As medications are introduced into the system, the body has devised its own mechanisms to eliminate them safely without causing toxic accumulation in the tissue. Such mechanisms involve two main organs: the liver and the kidney.
Most medications, especially the smaller, water-soluble molecules get excreted by the kidney, which in the process gets exposed to high concentrations of the drug. The kidney occasionally suffers damage as a result – particularly with long-term use of pain killers. Certain antibiotics are excreted by the kidney. In those with diminished renal function, especially in older people, this excretion process is impaired and the drug builds up quickly in the body. Smaller doses should be used in such settings to prevent toxicity and also to prevent further kidney damage.
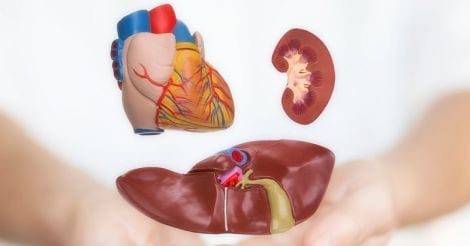
The liver handles heavier and fat-soluble molecules, initially breaking them down to smaller components through a complex set of chemical reactions, and then making them water-soluble by adding certain attachments to these fragments. Once the product is finally rendered harmless, it gets eliminated through bile into the intestine. The liver occasionally suffers damage by the very nature of this work.
The liver uses certain enzymes belonging to the cytochrome series to process these chemicals. Cytochromes are themselves a large family of enzymes that are located inside the microsomes inside each cell, and are prone to genetic variation. Within the super-family of cytochrome p450, there are six enzymes that account for the processing of 90 percent of all medications. A few people are born with genetic variations of the p450 enzymes, also called polymorphisms. These variations affect the way certain drugs are handled by the liver.
Certain cytochromes are earmarked for certain drugs, and when more than one drug competes for the same enzyme, the level of one or more drugs can rise abnormally in the body. As such incidents are uncommonly associated with symptoms, they may escape notice. Sometimes, toxicity becomes evident.
The above examples illustrate the importance of sound clinical knowledge and rational prescribing, without which medications can cause harm to unsuspecting patients. Self-medication is a major cause of liver and kidney disease worldwide.
Isn’t it better not to take medications?
Medications may cause side effects, but that doesn’t mean they should be avoided altogether. They are to be used judiciously to reduce human suffering, after analyzing the pros and cons in each case. The process of writing a prescription involves complex medical decision making. This requires in-depth knowledge of human physiology, biochemistry, pharmacology, pathology, microbiology, immunology and medicine, in addition to astute analytical and diagnostic skills.
Read: Everyday Health | How to make the most out of a doctor visit
Even in tightly regulated healthcare systems like the United States, the number of patients who suffer adverse drug reactions is substantial. This large reported number is because of diligent monitoring, documentation and reporting, a system that is largely absent in developing countries. Unfortunately, in India, even prescription medications are available over-the-counter and patients frequently consume them on their own or at the advice of unqualified people. While the actual number of such adverse events could be greater in India, the lack of awareness and paucity of reporting leads to a false sense of security that drugs are generally safe for self-use.
What can we do as patients to reduce the risk of experiencing side effects?
1. We must check with the doctor if the new medication is really necessary, as some medical conditions get better even without treatment. That said, there is nothing wrong with taking a mild pain killer for a splitting headache. This is because the suffering caused by the headache is far worse than the potential morbidity caused by the medication in an otherwise healthy person. At the other end of the spectrum are people who shun all medications due to irrational and disproportionate fear of side effects.
2. It is our duty to inform the doctor if we have had an adverse reaction with any medication in the past, and insist on that fact being displayed prominently in our medical record. As healthcare changes hands often, it is prudent to carry an updated list of our medications as well as our allergies in our wallet or handbag, and discuss them clearly with the doctor before the prescription is written.
3. Many people see more than one doctor for different conditions -- this frequently results in longer lists of medications. At each visit, we must update the doctor of all our medications, remembering to check each time if any of them can be discontinued to keep the list as short as possible.
4. Those patients who are on more than four medications are at much higher risk for drug interactions. It must be remembered that in India, many tablets combine two or more medications for convenience, and each of these must be counted separately.
5. Those with a family history or past history of a drug reaction are more likely to develop an adverse drug reaction. People with illnesses of the liver and kidney are also at greater risk. Among all medications, steroids, pain killers, blood-thinners and antibiotics are more frequently associated with side effects.
6. The elderly are especially at risk for side effects, due to several reasons. Their medication lists are longer, increasing the chance of drug interaction, and their metabolism is slower. Dosing might need to be modified, and medication lists kept trimmed to the extent possible in this group.
7. Many medications will have a long list of side effects, some of which will be exceedingly rare and impossible to remember. The doctor may mention only a couple of important side effects to watch out for, so that we don’t forget. For example, while prescribing steroids for a length of time, the doctor will ask us to keep an eye on our blood pressure and blood sugars.

8. Self-medication of prescription medications is to be avoided. There are medications in the OTC (over-the-counter) category that may be used for temporary symptomatic relief, but even an OTC medication like paracetamol, when used wrongly, can cause liver failure and death.
9. Those taking herbal and alternative medications too should report to the doctor. It is important to note that an herbal medication could contain numerous known and unknown active substances in contrast to a tablet in the system of modern medicine, which typically only has one active ingredient. As it is not conventional for herbal or alternative medications to list the side effects, there is a false public perception that there are ‘no side effects’ for anything of plant or alternative origin. In fact, herbal medications do cause side effects too, and the DILI network (drug induced liver injury) has published data on liver failure and deaths from unregulated use of herbal products worldwide.
Summary
In summary, there is no such thing as a 100 percent safe medication, regardless whether it is of plant or chemical origin. The term side-effect is man-made, and refers to effects of the medication in areas other than intended. As the body is made up of cells and organs that are connected with each other in many ways, it is obvious that if the medication has an effect, it has to have a side effect. Fortunately, the vast majority of the listed side effects and interactions do not occur in routine clinical practice. While most side effects are benign; a rare few are serious -- some of these can be fatal despite the best available treatment.
Even if the chance of a serious reaction is only one in 10,000 or 100,000 patients, it should be noted that for that patient and family, it is still a 100 percent.
The prevailing casual approach toward taking medications must be guarded against. Whether to treat, what to treat with, and how long to treat are decisions that must be taken by a person of sound knowledge and expertize, who has the patient’s safety and best interest in mind.
Further reading
1. List of pharmaco-vigilance centres in medical colleges of India.
http://www.ipc.gov.in/writereaddata/mainlinkfile/File414.pdf
2. Herbal medications causing liver toxicity
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4561772/
3. Hospitalization due to adverse drug reactions
https://www.ncbi.nlm.nih.gov/pubmed/18594048
4. US FDA’s module on preventable adverse drug reactions
5. The steps in drug development: from idea to approval
http://www.medscape.com/viewarticle/405869_4
6. The case report of a dangerous heart rhythm that eventually lead to the withdrawal of terfenadine
https://www.ncbi.nlm.nih.gov/pubmed/1977935
(The author is a Senior Consultant Gastroenterologist and the Deputy Medical Director, Sunrise Group of Hospitals, Kochi. His column, Everyday Health, will appear every alternate week)